19 hours ago3 Min Read. The vaccines cannot give you COVID-19.

Novavax Begins U S Phase 3 Trial Of Coronavirus Vaccine The Seattle Times
Novavax is conducting late-stage clinical trials for NVX-CoV2373 its vaccine candidate against SARS-CoV-2 the virus that causes COVID-19.
Novavax coronavirus vaccine phase 3. 22 hours agoIndonesia hard-hit by the coronavirus pandemic became the first country to approve the new vaccine for Covid-19 created by US-based Novavax the company announced Monday. On 24 February 2021 Novavax partnered with Takeda Pharmaceutical Company to manufacture the vaccine in Japan where its COVID-19 vaccine candidate is known as TAK-019. A final analysis of Novavaxs UK Phase 3 trial results announced in March showed that the vaccine had an overall efficacy of 897 -- and its efficacy was 964 against the original coronavirus.
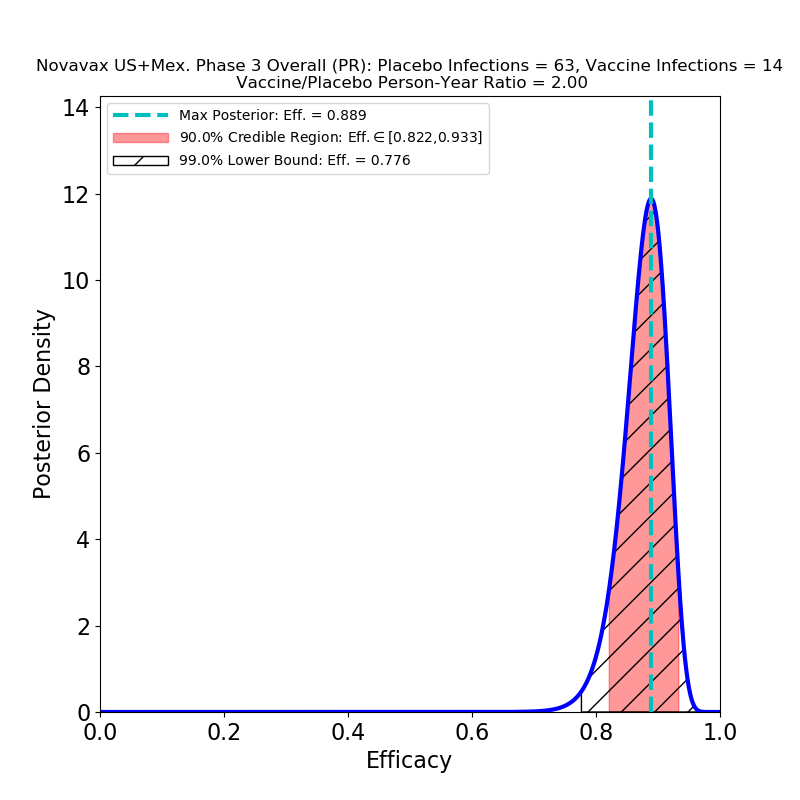
Reuters -Novavax Inc expects regulators in India the Philippines and elsewhere to make a decision on its COVID-19 vaccine within weeks its. Novavaxs two-dose COVID-19 vaccine showed 90 overall vaccine efficacy VE 100 protection against moderate and severe illness and 93 VE against variants of concern and of interest in a phase 3 US clinical trial in adults according to a company news release today. Lower risk of side effects may encourage vaccination among lower-income individuals who cant afford to miss a days worth of pay from work.
COVID-19 vaccine clinical trials including vaccines in earlier stages of development by visiting clinicaltrialsgov. Maryland-based Novavax plans to file for regulatory authorization for adults within the next few months the release said. 23 hours agoA vial of the Phase 3 Novavax coronavirus vaccine is seen ready for use in the trial at St.
Find information about the vaccine intended for vaccination providers. 1 day agoNovavax continues to deliver regulatory filings that we expect will bring the first protein-based COVID-19 vaccine based on Phase 3 data to the world said Stanley C. Georges University hospital in London Wednesday Oct.
Ad Safety is CDCs top priority and vaccination is a safer way to help build protection. Large-scale Phase 3 clinical trials are in progress or being planned for COVID-19 vaccines in the United States. Novavaxs COVID-19 vaccine has achieved 893 efficacy in a phase 3 clinical trial that enrolled subjects exposed to the B117 variant found in the UK.
The approval will give Indonesia which has strained to obtain adequate supplies of coronavirus vaccine for its 270 million people first access to the. 22 hours agoNovavax continues to deliver regulatory filings that we expect will bring the first protein-based COVID-19 vaccine based on Phase 3 data to the world said Stanley C. Based on Novavaxs phase 3 clinical trials their COVID-19 vaccine appears to have a substantially lower rate of side effects than the Pfizer-BioNTech or Moderna vaccines.
However the vaccine performed far. The Phase 3 trial of another investigational coronavirus disease 2019 COVID-19 vaccine has begun enrolling adult volunteers. Novavax continues to deliver regulatory filings that we expect will bring the first protein-based COVID-19 vaccine based on Phase 3 data to the world said Novavax Chief Executive Stanley Erck.
More than a year after its emergence as a global pandemic severe acute respiratory syndrome coronavirus 2 SARS-CoV-2. 11 hours agoIndonesia hard-hit by the coronavirus pandemic became the first country to approve the new vaccine for COVID-19 created by United States US-based Novavax the company announced Monday. The randomized placebo-controlled trial will enroll approximately 30000 people at approximately 115 sites in the United States and Mexico.
NanoFlu its quadrivalent influenza nanoparticle vaccine met all primary objectives in its pivotal Phase 3 clinical trial in older adults. Vaccines in Phase 3 Clinical Trials. Promising coronavirus vaccine news has dominated news coverage lately but one company just announced the second delay of a Phase 3 trial.
Erck president and. On 3 May 2021 Novavax initiated a pediatric expansion for the phase III clinical trial with 3000 adolescents 1217 years old. A COVID-19 vaccine developed by Novavax was had an overall efficacy rate of 90 and provided 100 protection against moderate and severe coronavirus cases in a Phase 3.
The Phase 3 trial of another investigational coronavirus disease 2019 COVID-19 vaccine has begun enrolling adult volunteers. To learn more about US. QUICK TAKE NVX-CoV2373 Covid-19 Vaccine 0230.
Biotechnology company Novavax has announced the long-awaited results from its large Phase 3 COVID-19 vaccine trial revealing over. The randomized placebo-controlled trial will enroll approximately 30000 people at approximately 115 sites in the United States and Mexico. They will help keep you from getting COVID-19.
It will evaluate the safety and efficacy of NVX-CoV2373 a vaccine candidate. Erck President and Chief. Ad See required Emergency Use Authorization EUA and safety information.

Novavax Covid 19 Vaccine Found To Be 96 4 Effective In Final Analysis Of Uk Phase 3 Study
Novavax Covid 19 Vaccine Trial Opens At Ut Health San Antonio University Health Ut Health San Antonio

Novavax Crows Over High Covid Vaccine Efficacy In Adults Medpage Today
Study Finds Novavax Covid 19 Vaccine About 90 Effective Kare11 Com
Novavax Expresses Fresh Confidence In Its Vaccine Politico

Uk To Offer New Vaccine Shots To Novavax Trial Volunteers News10 Abc

2020 Archive University Of Maryland School Of Medicine Begins Phase 3 Trial Of Novavax Covid 19 Vaccine Candidate University Of Maryland School Of Medicine
Serum Institute Applies For Bridging Trial Approval For Novavax Covid Vaccine Latest News India Hindustan Times
Studies The University Of Washington Virology Research Clinic
/cdn.vox-cdn.com/uploads/chorus_asset/file/22268097/AP21028822532028.jpg)
More Americans Received One Dose Of Covid 19 Vaccine Than Have Tested Positive Deseret News

Novavax Raleigh Couple Takes Part In Novavax Covid 19 Trial As Company Hopes For Emergency Authorization Abc11 Raleigh Durham

News Novavax Covid 19 Vaccine 89 3 Effective Nihr
Novavax Says Covid 19 Vaccine Shows 90 4 Overall Efficacy In Us Mexico Phase 3 Trial Abc11 Raleigh Durham
Novavax Vaccine Efficacy Data Promising Trials In Advanced Stage Centre Latest News India Hindustan Times

Novavax Covid 19 Vaccine Found To Be 96 4 Effective In Final Analysis Of Uk Phase 3 Study
Novavax Is Close To Finalising A Covid 19 Vaccine Supply Deal With The Eu Pmlive

Novavax Announces Results From Another Nvx Cov2373 Phase 3 Trial Covid 19 Vaccine Efficacy Page



ConversionConversion EmoticonEmoticon